Finasteride was one of the first treatments that the Food and Drug Administration approved as a hair loss drug. And for a long time, people believed it was a safe, easy treatment. All you needed was one pill a day and you’d grow your hair back.
For many people, finasteride remains a quick and easy hair loss treatment. But an increasing amount of people — particularly young people — are reporting serious side effects related to the use of this drug. These side effects, which are collectively known as post-finasteride syndrome, may linger for years, even after you stop taking this medication.
Finasteride: A DHT-blocking hair loss treatment
Finasteride is one of three FDA-approved treatments for androgenic alopecia (commonly known as male pattern hair loss). According to StatPearls Publishing, it was first approved as a treatment for benign prostatic hyperplasia (BPH) in 1992. A few years later, in 1998, it was approved at a lower dose to counteract male pattern hair loss.
Finasteride works as a DHT (dihydrotestosterone blocker). It essentially reduces DHT levels in men’s bodies to help prevent the progression of androgenic alopecia and counteract further hair loss.
What is a DHT blocker and why would I take one?
DHT is a hormone known as an androgen. It’s a perfectly normal hormone that is important and essential for human development. However, it’s also the primary hormone involved in androgenetic alopecia.
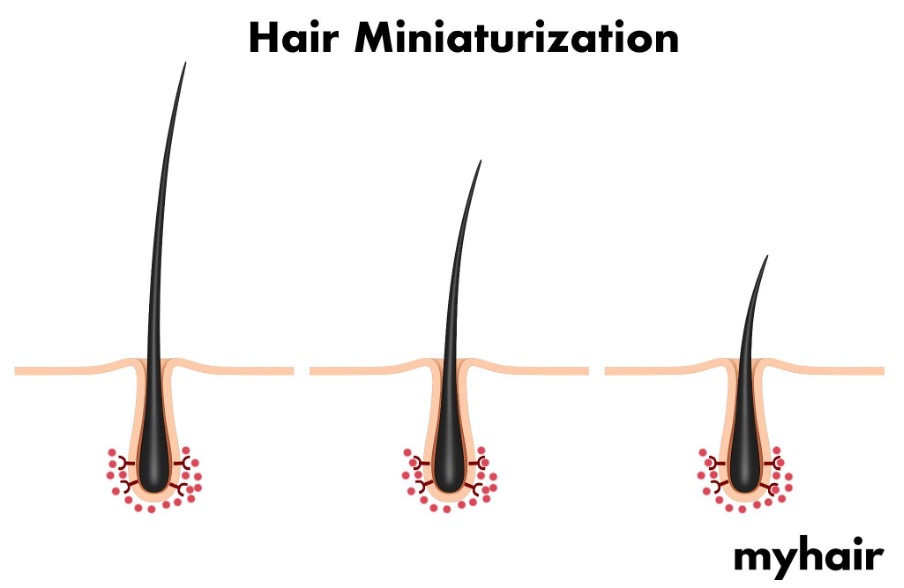
In some people, DHT binds to hair follicles. When this happens, their hair follicles start to shrink — a process known as miniaturization. This, in turn, causes thinner, weaker hair strands to be produced. Eventually, the hair follicles stop producing hair altogether. Blocking DHT can help prevent this succession of events — and consequent hair loss — from occurring.
Finasteride isn’t the only DHT blocker that exists. There are other DHT-blocking medications, like dutasteride (a stronger DHT blocker that’s commonly known as Avodart®), and natural DHT blockers, like saw palmetto. Finasteride, however, is the only DHT blocker sold as an FDA-approved treatment for androgenic alopecia.
Finasteride has been extensively studied and is definitely effective in treating pattern hair loss. However, it’s also become infamous for its side effects, particularly those related to sexual dysfunction. The most contentious of these side effects is known as post finasteride syndrome.
What is post-finasteride syndrome?
Finasteride is well known for having some unpleasant side effects. But in the last decade or so, various other issues have been reported. This cluster of side effects are referred to as post finasteride syndrome.
“Post-finasteride syndrome (PFS) is a constellation of serious adverse side effects manifested in clinical symptoms that develop and persist in patients during and/or after discontinuing finasteride treatment in men with pattern hair loss (androgenetic alopecia) or benign prostatic hyperplasia.“
A report published in the BMJ stated that post-finasteride syndrome symptoms include:
- Libido loss
- Erectile dysfunction
- Ejaculatory disorders
- Skin rash
- Gynaecomastia
- Fatigue
- Muscle weakness
- Hearing defects
- Metabolic anomalies
- Self harm
- Memory impairment
- Slow cognition
- Depression
- Suicidal ideation
- Anxiety
- Change in emotional affect
- Insomnia
What makes these side effects particularly concerning is that they seem to occur or continue even after people stop taking this medication. And to add to the complication, there isn’t thought to be a cure for post finasteride syndrome.
Is post-finasteride syndrome real?
Post-finasteride syndrome is extremely controversial. An entire foundation (the Post Finasteride Syndrome Foundation) was set up and dedicated to this condition in 2012.
Over 2,500 people with this issue have contacted the foundation since it was created. The majority are young people in their late teens to 30s.
The Post Finasteride Syndrome Foundation says that some — not all — men who take DHT-blocking medications like finasteride and dutasteride go on to develop post finasteride syndrome. The condition is meant to be rare, perhaps affecting around or less than 1 percent of users. The likelihood of experiencing post-finasteride syndrome is thought to increase based on the duration of time that you’ve taken the medication.
“Unfortunately, PFS is still not known or recognized by the large majority of all medical professionals, and many doctors cruelly stigmatize patients.“
At the moment, not all doctors and research acknowledge that post finasteride syndrome is real. For example, in 2019, researchers from Switzerland and Brazil recently referred to it as “An Induced Delusional Disorder with the Potential of a Mass Psychogenic Illness” in the Skin Appendage Disorders journal.
But an increasing number of researchers and medical professionals are at least starting to become open to the idea. That same year, the Aging Male journal released an article titled, “Post-finasteride syndrome – does it really exist?”, that discussed whether or not post finasteride syndrome and other DHT-blocker-associated side effects were real. Ultimately, their conclusion was that these reported side effects should be taken seriously, even if they affect only a small percentage of users.
“Despite the lack of final proofs, the presence of severe and persistent side effects caused by the treatment of an aesthetic problem raises great concern for the clinician. A low estimated prevalence of PFS cannot be an excuse for non-vigilance since the drug is used by millions of relatively young and healthy individuals.“
Post finasteride syndrome recovery
In general, once you stop taking a medication, any side effects associated with it should go away. This isn’t always instantaneous — it can take a bit of time. But eventually, the side effects should stop.
One of the biggest issues with post finasteride syndrome is that it may never go away. The Post Finasteride Syndrome Foundation says that this condition has no cure, and that few treatments are available.
Does this mean finasteride side effects are permanent? Not necessarily. A study in PeerJ – Life and Environment reported that one post-finasteride syndrome side effect, erectile dysfunction, went away eventually — but it took over 4 years.
Mental health issues associated with post finasteride syndrome
It is unclear when other post finasteride syndrome side effects, like depression, anxiety, and fatigue, go away. It’s also unclear in what percentage of people they remain a constant problem, or how many people choose to end their life as a consequence of these symptoms.
The Post Finasteride Syndrome Foundation has currently reported nearly 90 suicides associated with this condition. A recent study in JAMA Dermatology also touched on this sensitive subject, reporting that a disproportionate number of younger finasteride users (under 45) were likely to experience anxiety, depression, and suicidal ideation. This result is notably consistent with the Post Finasteride Syndrome Foundation’s findings: it’s mostly younger men that are affected by this condition.
Finasteride side effects acknowledged by the FDA
If you’re interested in taking finasteride and aren’t that concerned by post finasteride syndrome, you should still be aware of its side effects. Finasteride side effects are well established and acknowledged by the FDA. It’s just post finasteride syndrome that’s contentious and less well known.
If you’ve already purchased finasteride, chances are that you’ve either bought a generic version of this pill or gotten a drug called PROPECIA®. PROPECIA® is just the Merck & Co. brand name for finasteride. When prescribed for androgenic alopecia, this drug is taken in 1 milligram doses once a day.
Finasteride’s label and information pamphlet, which has been approved by the FDA, was most recently updated in 2012. Whether it’s a generic or PROPECIA®, each label will contain a warning, followed by a statement regarding potential adverse reactions. Below you can read the finasteride warnings and adverse reaction statement that comes with your medication:
Finasteride warnings
Finasteride is for men, so the first few statements about this medication are not surprising.
- “PROPECIA is not indicated for use in women or pediatric patients.”
- “Women should not handle crushed or broken PROPECIA tablets when they are pregnant or may potentially be pregnant due to potential risk to a male fetus.”
- “PROPECIA causes a decrease in serum PSA levels. Any confirmed increase in PSA* while on PROPECIA may signal the presence of prostate cancer and should be evaluated, even if those values are still within the normal range for men not taking a 5a-reductase inhibitor.”
- “5a-reductase inhibitors may increase the risk of high-grade prostate cancer.”
*PSA refers to a protein known as a prostate-specific antigen. According to the NIH National Cancer Institute, this protein is produced by the prostate gland. When PSA levels rise, it can indicate an enlarged prostate, inflammation of the prostate, a urinary tract infection, or prostate cancer.
Finasteride adverse reactions
According to the finasteride label and documentation:
“The most common adverse reactions, reported in ≥1% of patients treated with PROPECIA and greater than in patients treated with placebo are: decreased libido, erectile dysfunction and ejaculation disorder.”
Other adverse reactions are mentioned, but the documentation specifically states that: “the incidences for breast tenderness and enlargement, hypersensitivity reactions, and testicular pain in finasteride-treated patients were not different from those in patients treated with placebo.” This means that only libido, erectile dysfunction, and ejaculation problems are considered to be likely problems for people taking finasteride for hair loss.
Other finasteride adverse reactions
Finasteride can also have other potential side effects, but these are only stated to be relevant to taking higher doses of finasteride (5 milligrams). This is the recommended dose for people taking this medication for an enlarged prostate.
According to the FDA-approved documentation, these additional adverse reactions include:
- Impotence
- Breast enlargement
- Breast tenderness
- Rash
In addition to these adverse reactions, other side effects have also been reported. However, these are considered to be voluntarily reported adverse reactions, reported from a “population of uncertain size”. This means that the label can’t say the exact number of people — whether it’s 0.1 percent, 1 percent, 10 percent, or any other specific amount — that are likely to experience these other issues when taking finasteride.
The less common finasteride side effects that have been reported after taking this drug and acknowledged in this label include:
- Hypersensitivity reactions, like rashes, hives, itchiness, or swelling of the lips and face
- Reproductive system disorders, including sexual dysfunction that continued after discontinuation of treatment such as erectile dysfunction, libido disorders, ejaculation disorders, and orgasm disorders; male infertility and/or poor seminal quality, and testicular pain.
- Breast disorders, including male breast cancer
- Depression
So, is finasteride safe?
The FDA considers finasteride to be a safe and effective drug. There have been countless clinical trials reviewing the efficacy of this medication and its side effects.
Despite the controversy associated with post finasteride syndrome, it’s important to remember that these side effects are considered to be rare. Even the Post Finasteride Syndrome Foundation admits that many people experience no side effects after using finasteride.
However, given the risk and severity of the reported side effects, finasteride may be a hair loss treatment most appropriate for older men, who seem to be at lower risk for these issues. Minoxidil, a different FDA-approved medication for androgenic alopecia, may be a better choice for younger people.
It’s also important to realize that the above listed issues aren’t necessarily restricted to finasteride. Dutasteride, which is sold as a treatment for androgenic alopecia in South Korea and Japan, is also capable of causing these side effects. In fact, almost any DHT blocker could, in theory.
But wait — don’t go swearing off all DHT blockers because of this. You should know that caffeine and various other common edible compounds are also DHT blockers. They’re simply a lot weaker than medications like finasteride or dutasteride, and therefore very unlikely to cause the same types of reported issues.
Are any other DHT blockers safer?
There aren’t any alternative, safer DHT blockers currently approved as hair loss treatments by the FDA. However, there are various types currently being studied.
One alternative that seems to be working well is topical finasteride. According to a study in the Journal of Drugs in Dermatology, users of topical finasteride don’t report the majority of the same symptoms that oral finasteride users do.
The main overlapping symptom between oral and topical finasteride, which was rarely reported, was testicular pain. Instead, topical finasteride users report milder side effects, like dermatitis, skin irritation, headaches, increased liver enzymes, lightheadedness, sore throat, and bed-wetting.
Eventually, topical finasteride may be sold in a pharmacy near you. There’s a good chance that it might even be sold mixed with minoxidil, too. This combination treatment is also being studied and is showing promising results.